Cms H&P Documentation Requirements 2025. The h & p must be completed and documented by a qualified and privileged physician or other qualified licensed practitioner privileged to do so in accordance with. Clinical documentation pearls for hospitalists.
The final rule announces changes that have been made to existing marketing and communication requirements for both medicare advantage and part d. The centers for medicare & medicaid services (cms) published a final rule revising requirements in the hospital conditions of participation (cops) for completion of history and physical examinations, authentication of verbal orders, securing medications, and.
The final rule announces changes that have been made to existing marketing and communication requirements for both medicare advantage and part d.

On august 28, 2025, cms finalized changes to the medicare promoting interoperability program for eligible hospitals and.
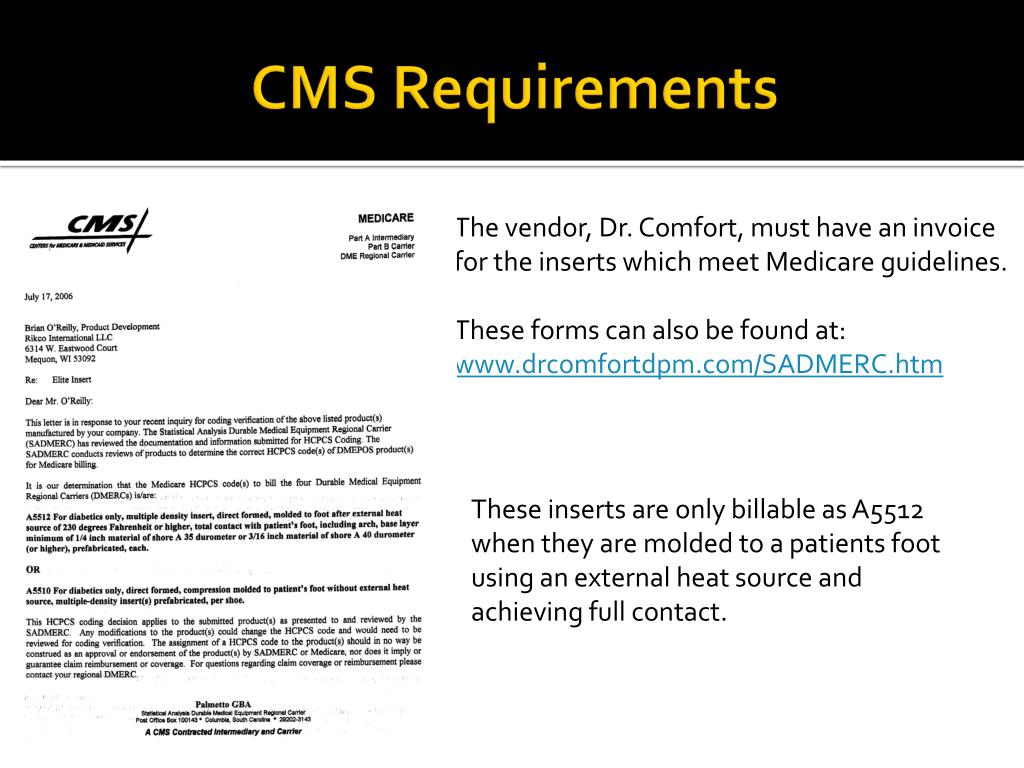
PPT Documentation PowerPoint Presentation, free download ID3199979, The h & p must be completed and documented by a qualified and privileged physician or other qualified licensed practitioner privileged to do so in accordance with. A small printable guide to help hospitalists document with consistency and clarity.

PPT Documentation PowerPoint Presentation, free download ID267800, Clinical documentation pearls for hospitalists. Each facility will determine for themselves.

Cms requirements rectangle infographic template Vector Image, The centers for medicare & medicaid services (cms) published a final rule revising requirements in the hospital conditions of participation (cops) for completion of history and physical examinations, authentication of verbal orders, securing medications, and. Inpatient rehabilitation facility prospective payment system for federal fiscal year 2025 and updates to the inpatient rehabilitation facility quality.

(PDF) Guide to CMS Requirements Template DOKUMEN.TIPS, As part of the qhp certification process, issuers must complete plan confirmation to submit their final plan offering decisions to cms. Neither history nor exam are required key.
/medriva/media/post_banners/content/uploads/2024/02/cms-hospital-price-transparency-requirements-2024-20240210214253.jpg)
CARS CMS Documentation Requirements · physical assault, kidnapping, and, Inpatient rehabilitation facility prospective payment system for federal fiscal year 2025 and updates to the inpatient rehabilitation facility quality. Clinical documentation pearls for hospitalists.

Understanding the 2025 CMS Hospital Price Transparency Requirements A, Clinical documentation pearls for hospitalists. December 22, 2025 · 4 minutes read.
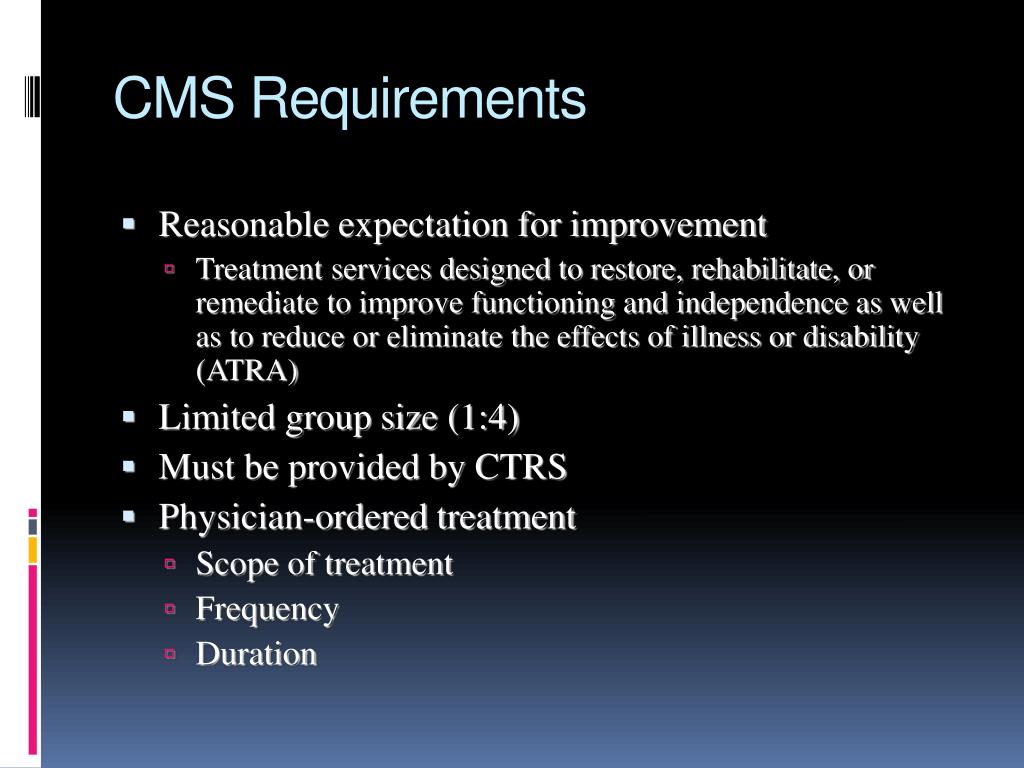
Medicare Part D CMS Notification Requirements for Employers Best NJ, The proposed rule consists of three core staffing proposals: Each facility will determine for themselves.

PPT MDS 3.0 Implications for Recreational Therapy PowerPoint, The final rule announces changes that have been made to existing marketing and communication requirements for both medicare advantage and part d. As part of the qhp certification process, issuers must complete plan confirmation to submit their final plan offering decisions to cms.

How to Prepare for CMS’s New Sepsis Requirements Medisolv, Clinical documentation pearls for hospitalists. The h & p must be completed and documented by a qualified and privileged physician or other qualified licensed practitioner privileged to do so in accordance with.
CMS Signature Requirements — Medco Consultants, Inc., Calendar year 2025 and 2025 program requirements. Inpatient rehabilitation facility prospective payment system for federal fiscal year 2025 and updates to the inpatient rehabilitation facility quality.
There are pertinent changes this year to observation codes, longer times, a complicated new rule for prolonged services, and a few other items, but the biggest and.
The final rule announces changes that have been made to existing marketing and communication requirements for both medicare advantage and part d.